The Importance, Properties, And Applications Of Sulfuric Acid
H2S04, or sulfuric acid, is one of the most important industrial chemicals in the world. It plays a critical role in various processes, from manufacturing fertilizers to petroleum refining. Understanding the properties, uses, and safety measures associated with sulfuric acid is essential for industries and individuals alike. In this article, we will delve deep into H2S04 to provide a comprehensive overview of its significance, handling, and applications.
As a highly versatile compound, sulfuric acid (H2S04) is a strong mineral acid that can be utilized in multiple sectors, including agriculture, manufacturing, and chemical synthesis. Its high reactivity and ability to act as both an acid and dehydrating agent make it indispensable. This article aims to explore the multifaceted nature of H2S04, highlighting its essential characteristics and its role in various applications.
Moreover, given the potential hazards associated with sulfuric acid, it is crucial to comprehend the safety precautions and environmental considerations related to its use. By the end of this article, readers will have a thorough understanding of H2S04, its properties, its applications, and the necessary safety measures to consider when handling this powerful acid.
Table of Contents
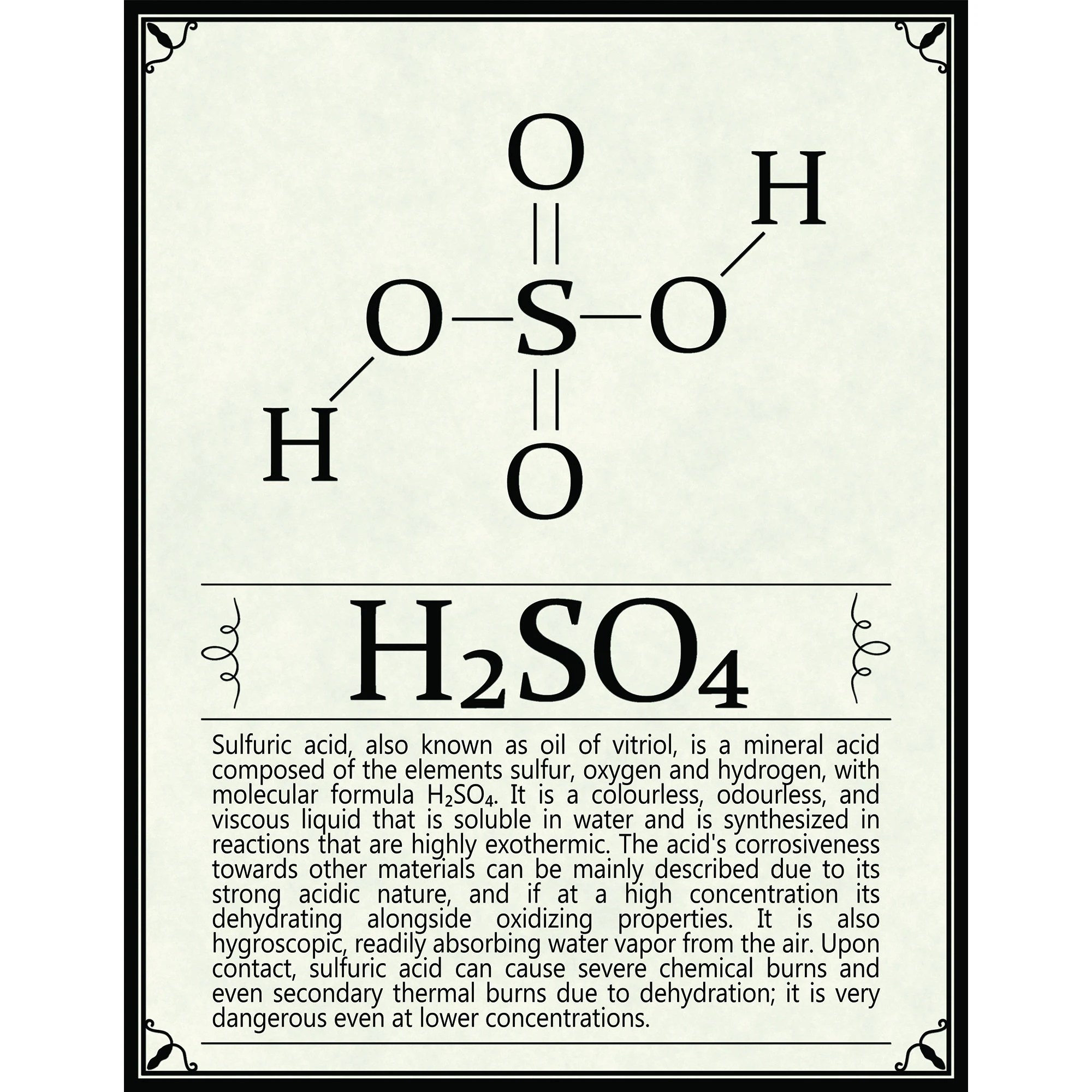
What is H2S04?
H2S04, commonly known as sulfuric acid, is a colorless, odorless, and oily liquid that is highly corrosive and reactive. It is classified as a strong acid due to its ability to completely dissociate in aqueous solutions, releasing hydrogen ions (H+) and sulfate ions (SO42-). This property makes H2S04 extremely useful in various chemical reactions and industrial applications.
Historical Background
The history of sulfuric acid dates back to the 8th century when it was first discovered by the Persian alchemist Jabir ibn Hayyan. It was later produced in larger quantities during the 17th century, leading to its widespread use in chemical processes. Today, sulfuric acid is one of the most produced industrial chemicals globally, with millions of tons manufactured each year.
Properties of H2S04
Understanding the properties of H2S04 is essential for its safe handling and effective application. Here are some key properties:
- Molecular Formula: H2S04
- Molar Mass: 98.079 g/mol
- Density: 1.84 g/cm3
- Boiling Point: 337 °C (639 °F)
- Melting Point: 10 °C (50 °F)
- Solubility: Highly soluble in water, releasing heat upon dissolution
Production of H2S04
The production of sulfuric acid primarily involves the contact process, which includes the following steps:
Applications of H2S04
Sulfuric acid is utilized across various industries, showcasing its versatility:
- Fertilizer Production: H2S04 is essential in producing phosphoric acid, which is a key ingredient in fertilizers.
- Petroleum Refining: It is used to remove impurities and improve the quality of refined petroleum products.
- Chemical Manufacturing: Sulfuric acid is a crucial reactant in manufacturing various chemicals, including hydrochloric acid and nitric acid.
- Metal Processing: H2S04 is used in pickling metals to remove rust and scale.
Safety Measures for H2S04
Given its corrosive nature, handling sulfuric acid requires strict safety measures:
- Always wear personal protective equipment (PPE), including gloves, goggles, and face shields.
- Store sulfuric acid in a cool, dry place, away from incompatible materials.
- Ensure proper ventilation in areas where H2S04 is used or stored.
- Have neutralizing agents (such as sodium bicarbonate) readily available in case of spills.
Environmental Impact of H2S04
The environmental impact of sulfuric acid is significant, particularly concerning its release into the atmosphere:
- Acid Rain: Sulfuric acid can contribute to acid rain, which adversely affects ecosystems, soil, and water sources.
- Soil Degradation: Spills or leaks can lead to soil contamination, affecting plant growth and soil health.
Future of H2S04
As industries evolve, the demand for sulfuric acid is expected to grow, particularly in sustainable practices. Innovations in production methods and applications are likely to emerge, focusing on minimizing environmental impact while maximizing efficiency.
Conclusion
In conclusion, H2S04, or sulfuric acid, is a vital chemical with wide-ranging applications across various industries. Understanding its properties, production methods, and safety measures is crucial for those working with or studying this compound. As we advance, it is essential to balance its benefits with responsible environmental practices.
We encourage you to leave a comment or share this article if you found it informative. For more articles on related topics, feel free to explore our website.
ncG1vNJzZmivmaC2b7XSrJirrZKWe6S7zGikmrCemsS0g46haaxoZGO1tbnL